Reading Apprenticeship Inspired Assignment or Lesson
Reading Apprenticeship Inspired Assignment or Lesson
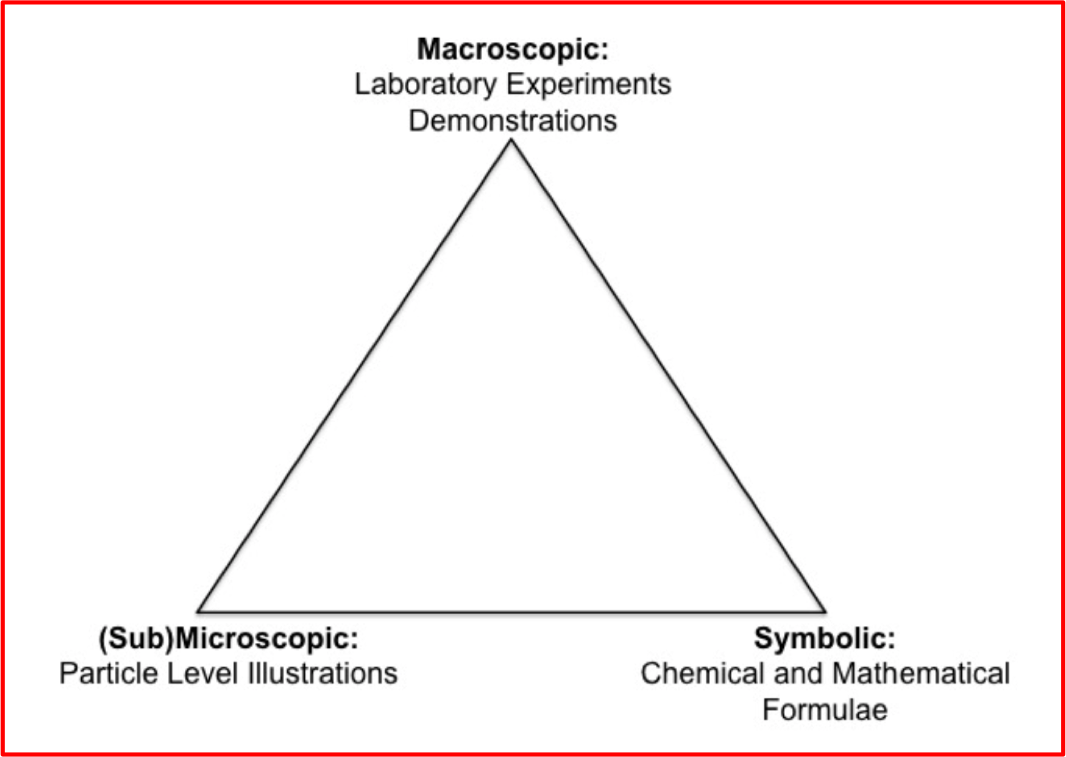 Johnstone's Triangle includes 3 levels of representation
Johnstone's Triangle includes 3 levels of representation
This activity is designed to take place at the beginning of coverage of equilibrium in a second semester general chemistry course. It does not rely on the students having covered kinetics, so there is no reference to a definition of equilibrium often found in general chemistry texts, where a cehmical system is defined as being at equilibrium when the rate of the forward reaction is equal to the rate of the reverse reaction. In my course, I am using these activies about a third through the semester; the students have:
- submitted a personal STEM reading history;
- developed a strong social environment;
- developed a reading strategies list,
- been using Think-Pair-Share, Talk to the Text, Thi)nk Aloud, Talk Aloud Paired Problem Solving (TAPPS, and reading logs as semester long routines.
The students' understanding is assessed in several ways:
- During an in-class activity, the instructor can monitor students' understanding by moving from group to group, listening to the students' conversations, reading their responses to the questions, and how they construct the additional plots asked for.
- For a synchronous on-line activity, the instructor can also monitor student understanding by visiting breakout rooms to monitor the discussion, ask questions, etc. The student may also be asked to submit their work.
- In an asynchronous setting, the students may be asked to submit their work as well as participate in a discussion.
This activity asks students to apply prior knowledge to a new situation, and to surface their disiplinary thinking and problem solving processes. it also supports students' understanding of how scientific information can be represented, in this situation, as a graph.
This activity was originally designed as an in-class activity; it can transition to synchronous as well as asynchronous, with some modification to include discussion posts.
Preread from the OpenStax Chemistry text: Chapter 13, section 13.1 , or other resources such as LibreText.
The graph and worksheet are found here.
This activity is a modification of one from "Water Treatment: How Can We Make our Water Safe to Drink? ", by Susan E. Kegley , Doug Landfear , David Jenkins , Kome Shomglin ISBN-13: 978-0393926460


