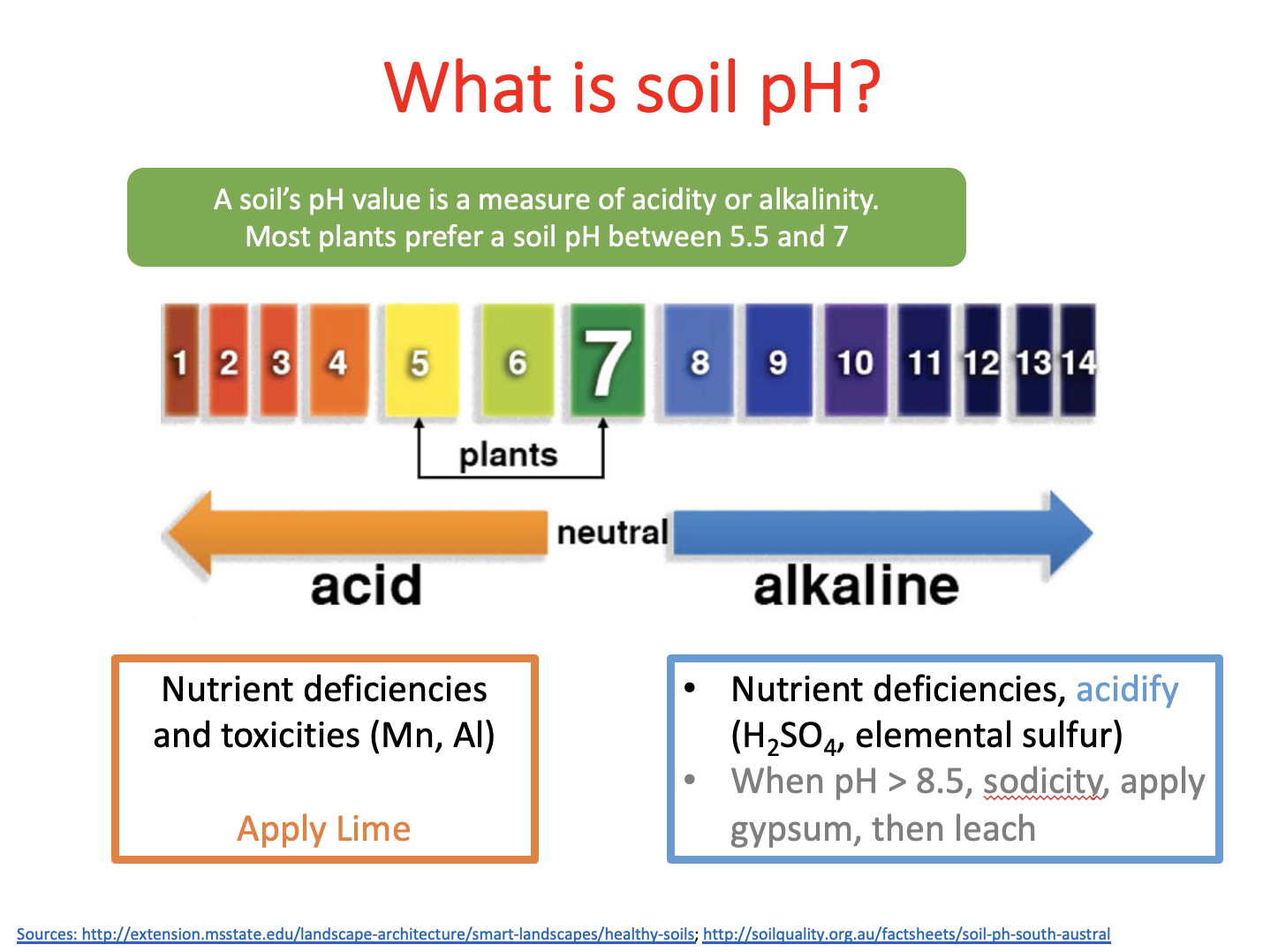
Lab 3 pH and salinity
Lab 3 pH and salinity
- If the H+ concentration is 0.00023 moles/L, what is the pH? Show your work!
- If the H+ concentration is 0.00001 moles/L, what is the OH- concentration in moles/L? Show your work!
- If the pH of your soil was 6.2, what general conclusions can you make concerning the likelihood that you need to apply lime to your soil?
- Describe 3 ways pH can affect plant health
- Describe 2 reasons why soil pH changes and varies between soils
There are generally three pools of acidity in the soil: active pH, salt-extractable or exchangeable acidity and reserve acidity. Watch the video below the learn the difference, and why it matters.
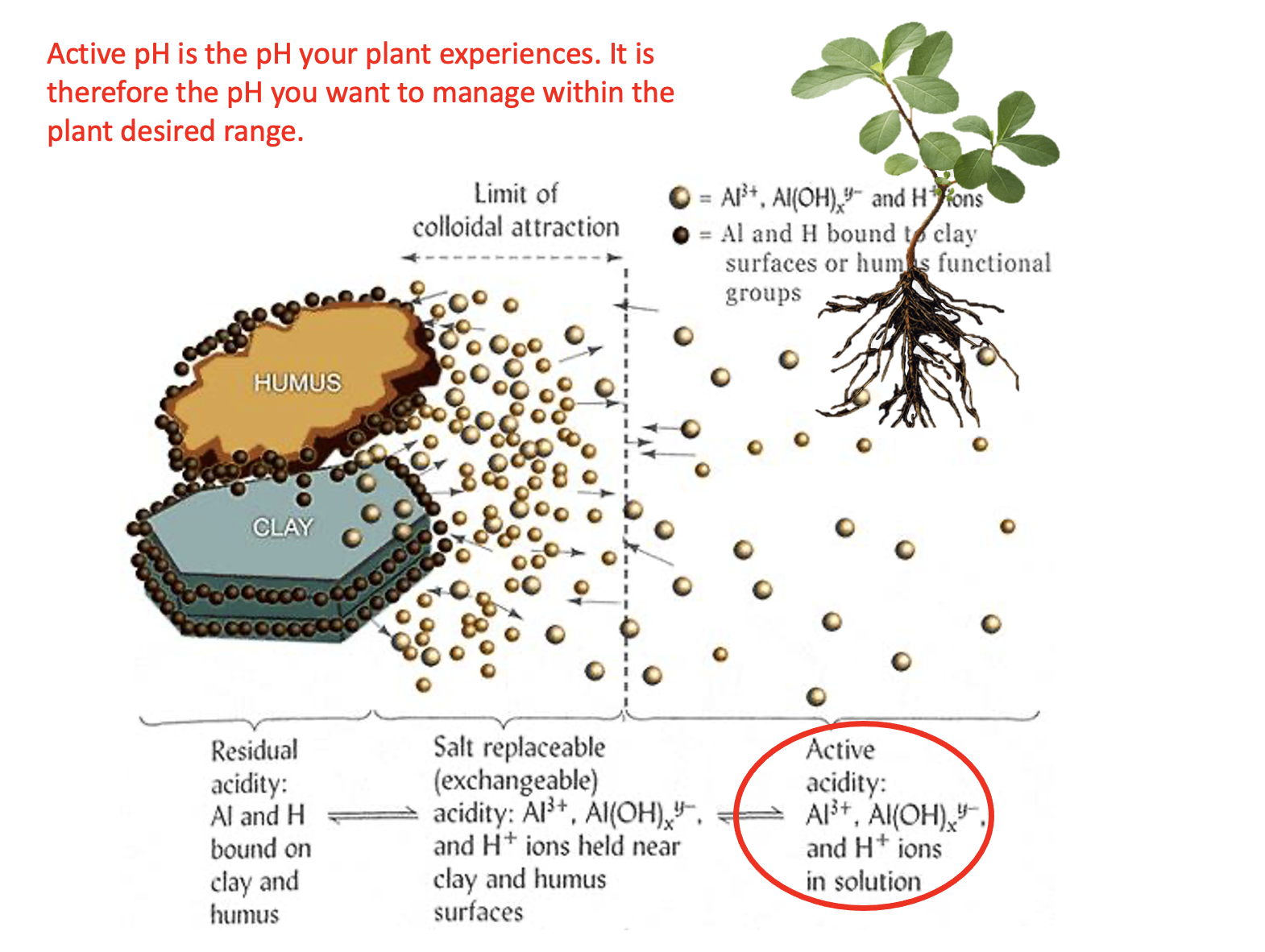
- Match pools of acidity with the method of measurement
Measurement methods possible answers: KCl extraction and titration; pH probe in water:soil solution; Cannot be easily determined directlyPool of acidity Measurement method Active pH Salt Exchangeable pH Residual pH - Explain why Al3+ is considered an acid cation
- Which of the following statements is correct?
- The greater the amount of acidity associated with the exchange complex on the colloids, the smaller the soil's buffer capacity
- The greater the buffering capacity of the soil, the less liming material is required to neutralize the soil acidity
- The greater the buffering capacity of the soil, the more liming material is required to neutralize the soil acidity
- Which pool of soil acidity do you want to keep within the plant's desired pH range?
- Active acidity
- Salt exchangeable acidity
- Residual acidity
Acid soils have been noted in areas of northern and central California. These soils tend to be moderately to highly weathered, or poorly buffered and acidified through nitrogen fertilization.
The lime requirement of a soil is the amount of lime or other base required to neutralize soil acidity from the initial acid condition to a selected less acid condition. It is defined in terms of pure lime (CaCO3) because CO3- is the most common base used for neutralizing soil acidity.
The method used to determine the lime requirement for a soil depends on the type of soil acidity present to be neutralized (H+, Al3+ and hydroxyl-Al). The most common method used by labs in California is the Shoemaker, McLean, and Pratt (SMP) Single Buffer Method. The method is based on the reaction of soil acidity with a chemical buffer, resulting in change in the pH of the buffer.
The SMP buffer is added to a soil-water slurry after measurement of soil pH. The SMP buffer has an initial pH of 7.5. Bases in the buffer react with soil acidity to reduce the pH of the buffer from 7.5 to some measured “soil-buffer” pH. The greater a decline in soil-buffer pH from an initial value of 7.5, the greater the lime requirement for neutralizing the acid in the soil. The amount of lime required is estimated by the calibration of soil-buffer pH to amounts of lime required to achieve a target soil pH in soils incubated with varying amounts of pure CaCO3.
Table: Calibration for lime requirement for the surface 20 cm (8 inches) of soil using the SMP buffer pH method.
Amount of 100% pure CaCO3 required (tons/ac)
| Measured Soil SMP Buffer pH | Target pH = 7 | Target pH = 6.5 | Target pH = 6 |
| 6.8 | 1.1 | 0.9 | 0.8 |
| 6.7 | 1.8 | 1.6 | 1.3 |
| 6.6 | 2.4 | 2 | 1.7 |
| 6.5 | 3.1 | 2.6 | 2.1 |
| 6.4 | 4.0 | 3.4 | 2.8 |
| 6.3 | 4.7 | 4 | 3.3 |
| 6.2 | 5.4 | 4.6 | 3.7 |
| 6.1 | 6 | 5 | 4.1 |
| 6.0 | 5.8 | 5.7 | 4.7 |
| 5.9 | 7.7 | 6.5 | 5.3 |
| 5.8 | 8.3 | 7 | 5.7 |
| 5.7 | 9 | 7.6 | 6.2 |
| 5.6 | 9.7 | 8.2 | 6.7 |
| 5.5 | 10.4 | 8.8 | 7.2 |
How liming materials neutralize soil acidity
Note, it is the carbonate that neutralizes the pH. The only role of calcium is to move acid cations on the cation exchange complex into soil solution.
Bases attached to Ca2+ or Mg2+ in liming materials include:
- Carbonate: CO32-
- Oxide: O2-
- Hydroxide: OH-
- Bicarbonate: HCO3-
- Silicate: SiO32-
A liming material is characterized by its “neutralizing value”, which is expressed as Calcium Carbonate Equivalent (CCE), and by its “degree of fineness” (“Reactivity”).
Calcium Carbonate Equivalence
The CCE of a liming material is the acid-neutralizing capacity of the material expressed as a weight percentage of CaCO3
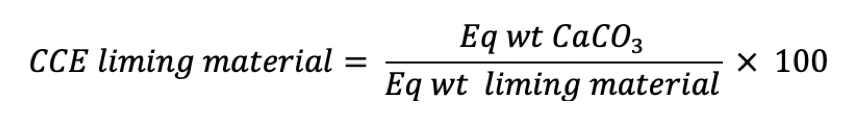
Relative neutralizing values of liming materials (CCE)
| Material | Relative neutralizing value, % |
| Calcium carbonate | 100 |
| Dolomitic lime | 95 – 108 |
| Calcitic lime | 85 – 100 |
| Baked oyster shells | 80 – 90 |
| Marl | 50 – 90 |
| Burned lime | 150 – 175 |
| Hydrated lime | 120 – 135 |
| Basic slag | 50 – 70 |
| Wood ashes | 40 – 80 |
Fineness
Effectiveness of agricultural liming materials depends on particle size distribution. Screen mesh sizes are designated by the number of openings per square inch, e.g. 8 mesh has 8 openings per sq inch (about ¼”); 60 mesh has 60 openings per sq inch.
Rules of thumb:
- Particles of greater than ¼ diameter dissolve too slowly to be effective
- Particles passing ¼” but not 60 mesh are 50 – 60% effective
- Particles passing 60 mesh – 100% effective
Effective Calcium Carbonate Equivalent
Effective calcium carbonate equivalent is a rating of the overall quality of a liming material. Effective CCE takes into account the quality and fineness factors in assessing a liming material.
ECCE = Calcium Carbonate Equivalent (CCE) x Fineness Factor
Where CCE is determined in a laboratory (acid neutralizing factor); Fineness Factor of a liming material is calculated as:
(% > 8-mesh screen x 0.0) + (% 8- to 60-mesh screen x 0.5) + (% < 60-mesh screen x 1.0)
Where % = percent of the weight of the liming material; 0.0, 0.5, and 1.0 are the relative effectiveness values for the different sizes of particles (based on solubility).
Example Calculation of ECCE for two liming materials
| Liming material A | Liming material B | |
| Calcium Carbonate Equivalent (CCE) (%) | 80 | 60 |
| % passing or retained on sieve | ||
| > 8 mesh | 18 | 0 |
| 8 - 60 mesh | 31 | 20 |
| < 60 mesh | 51 | 80 |
| Fineness Factor Calculation (FF) | ||
| > 8 mesh x 0 efficiency | 18 x 0 = 0 | 0 x 0 = 0 |
| 8 - 60 mesh x 0.5 efficiency | 31 x 0.5 = 16 | 20 x 0.5 = 10 |
| < 60 mesh x 1.0 efficiency | 51 x 1.0 = 51 | 80 x 1.0 = 80 |
| Total fineness factor (sum) | 67 | 90 |
| Effective Calcium Carbonate Equivalence (ECCE) = CCE x FF | 0.8 x 67 = 54 | 0.6 x 90 = 54 |
Calculation example
It has been determined that 2,000 lbs of pure CaCO3 is required to neutralize the acidity in the top foot of your soil. You have an inexpensive source of the Liming Material A in the table above. How many pounds of Liming Material A are required to neutralize the acidity?
2000 lbs pure CaCO3/0.54 = 3704 lbs of liming material A
- The SMP buffer pH of your soil is 5.6 and you want to raise your soil pH to 6.5. How many tons of pure CaCO3 would be required to treat 10 acres your soil to a depth of 1 foot. Show your work!
- Calculate the pounds of dolomitic lime [CaMg(CO3)2] per acre of soil you would need to apply if the recommendation indicated to apply 1800 lbs of pure CaCO3/acre. Use the table below to help you come to your final answer.
Dolomitic lime Calcium Carbonate Equivalent (CCE), % 95 % passing or retained on sieve > 8 mesh 10 8 - 60 mesh 25 < 60 mesh 65 Fineness factor calculation > 8 mesh x 0 efficiency 8 - 60 mesh x 0.5 efficiency < 60 mesh x 1.0 efficiency Total fineness factor (sum) Effective Calcium Carbonate Equivalent (ECCE), % = CCE x FF lbs/ac.ft of dolomitic lime required A farmer has two fields that require liming to the same final pH. The characteristics of the soils in each of these fields are indicated in the table below. He has lost the liming recommendation sent to him by the soil testing lab, but he remembers that 5 tons per acre was recommended for Field #B. Because the pH is the same in both fields, he applies 5 tons to Field #A also. Has he acted wisely? Explain!
Field A Field B Percent organic matter 1.1 3.5 Percent clay 15.2 38.1 Percent sand 74.0 51.2 Dominant type of clay 1:1 2:1 pH 5.5 5.5
Soil salinity is a common problem in California. Learn about why soil salinity occurs and how it affects agricultural production.
- Describe visual symptoms of salt stress in plants, described in the video
- Why do plants experience stress when soil salinity is high?
- Explain why soil salinity in California is expected to get worse with climate change?
- List 2 approaches that are mentioned in the video that could help address challenges related to soil acidity
Soil salinity is not only problematic in California, but affects many states in the USA, especially in drier regions. The general management strategy to mitigate a soil salinity problem is to leach the salts down with irrigation water or during seasons with high rainfall. Learn from an agricultural extension specialists in North Dakota how one can make sure leaching efforts to remove salts from the crops root zone can be made more effective.
- List 3 strategies growers can take to improve the chance that salts stay below the root zone
- In the video, we learn that besides soil salinity, soil sodicity can also occur. We will talk more in the lecture about the difference between soil salinity and sodicity, and why it matters. For now, remember in which case the video recommends applying gypsum prior to leaching the salts down.
- Salinity
- Sodicity
- Both salinity and sodicity
- None of the above
Soil salinity is assessed by electrical conductivity or EC. As we know, soil salinity is associated with the accumulation of salts in the soil solution. When salts are in solution, they dissociate into cations and anions. These are charged particles that will conduct electricity. Therefore, the greater the salinity, the greater the EC. In this video, you will learn how EC in the soil can be determined, and what important implications are of the methodology.
Plants have different levels of tolerance for salinity. To determine if your crop is at risk of yield loss due to soil salinity, the EC of the soil needs to be compared to the crop threshold level. For many crops, the EC level above which a yield loss of at least 10% is expected has been documented, as shown in the table below. Soil salinity (ECe) values for crops at which a 10% yield loss is expected. If the EC of your soil exceed the threshold level for the crop you want to grow, you will either need to leach the salts down, or choose to grow another crop that is less sensitive to salinity.
Table: Crop Tolerance and Yield Potential of Selected Crops as Influenced by Soil Salinity (ECe)
| Crop | ECe Tolerance for 90% Yield potential |
| FIELD CROPS | |
| Barley | 10 |
| Cotton | 9.6 |
| Sugarbeet | 8.7 |
| Sorghum | 7.4 |
| Soybean | 5.5 |
| Rice | 3.8 |
| Corn | 2.5 |
| Bean | 1.5 |
| Alfalfa | 3.4 |
| VEGETABLE CROPS | |
| Squash, Zucchini | 5.8 |
| Broccoli | 3.9 |
| Tomato | 3.5 |
| Cucumber | 3.3 |
| Celery | 3.4 |
| Cabbage | 2.8 |
| Potato | 2.5 |
| Sweet potato | 2.4 |
| Pepper | 2.2 |
| Lettuce | 2.1 |
| Onion | 1.8 |
| Carrot | 1.7 |
| FRUIT CROPS | |
| Grapefruit, Orange | 2.4 |
| Peach | 2.2 |
| Apricot | 2.0 |
| Grape | 2.5 |
| Almond | 2.0 |
| Plum | 2.1 |
| Strawberry | 2.0 |
Reference values in this table have been compiled by faculty and staff at Cal Poly throughout the years, and the original references for the reference values have been lost. For a more comprehensive list of reference values for a variety of crops including original sources, students are referred to the FAO Manual entitled Agricultural Drainage Water Management in Arid and Semi-Arid Areas, Annex 1.
- What is the benefit of using a saturated paste to determine soil salinity?
- How do you know that you have added enough water to your soil to obtain a saturated paste?
- Which of the crops listed in the table with EC threshold values is most sensitive to salinity?
When the build-up of soluble salts (from irrigation water and fertilizers) in the soil reaches levels that reduce crop yield, the salts can be leached by applying more water than that needed by the crop during the growing season. This extra water moves at least a portion of the salts below the root zone by deep percolation. The depth of water required for leaching depends on the salt sensitivity of the crop and the salinity of the applied water. Leaching can only be done, however, if the drainage below the crop root zone is sufficient to prevent a rise in the water table that will bring the leached salts back up into the root zone. When the salinity of the irrigation water is high, the depth of leaching water needed may be too great, making it necessary to change to a more salt tolerant crop.
Leaching is a basic step in production even for water of the best quality and must be practiced when necessary to avoid accumulation of salts from fertilizers that could ultimately affect production. The questions that arise are how much water should be used for leaching?
The leaching requirement
The leaching requirement is the portion of water applied which is required to pass through the root zone to control salts at a specific level. To estimate the leaching requirement, both the irrigation water salinity (ECw) and the crop tolerance to soil salinity (ECe) must be known. The table in the section 'diagnosing salinity' in this assignment contains an acceptable ECe value for each crop appropriate to the tolerable degree of yield loss (usually 10 percent or less). ECw is obtained from a water analysis.
The necessary leaching requirement (LR) can be estimated for a particular crop:
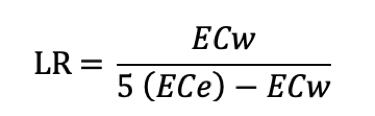
LR = minimum leaching requirement needed to control salts within the tolerance ECe of the crop with ordinary surface methods of irrigation; ECw = salinity of the applied irrigation water in dS/m; ECe = average soil salinity tolerated by the crop as measured on a soil saturation extract (dS/m). It is recommended that the ECe value that can be expected to result in at least a 90 percent or greater yield be used in the calculation.
The number obtained for the LR from the above equation is used to obtain an estimate of the amount of water required (total water applied to crop) on a seasonal basis for salinity control, using the following equation:
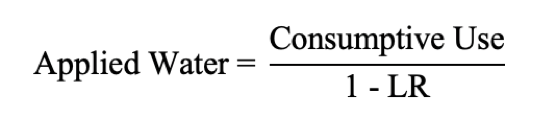
Consumptive Use is the total amount of water needed and is equivalent to the crop evapotranspiration (ETc). Values for ETc vary with crop, irrigation system, and climate. Note that if the ECw becomes close to five times the ECe, the amount of water needed to leach salts becomes unreasonable. Relatively high quality irrigation water is a prerequisite for this strategy to work.
Estimated Consumptive Water Use (in) for Crops in the Central Valley, California. Drip/Micro Irrigation Typical Year
| Estimated Consumptive Water Use (inches) | |
| Apple, Pear, Cherry, Plum, Prune | 42 |
| Peach, Nectarine, Apricot | 40 |
| Immature Peaches, Nectarines, etc. | 25 |
| Almonds | 42 |
| Almond with Cover Crop | 53 |
| Walnuts | 44 |
| Pistachio with Cover Crop | 51 |
| Cotton | 34 |
| Misc. Field Crops | 28 |
| Small vegetables | 19 |
| Tomatoes and peppers | 26 |
| Potatoes, Sugar beets, Turnips, etc. | 36 |
| Melons, Squash, Cucumbers | 18 |
| Onions and Garlic | 21 |
| Strawberries | 28 |
| Flowers, Nursery and Christmas Trees | 40 |
| Citrus (no ground cover) | 38 |
| Avocado | 40 |
| Msc Subtropical | 40 |
| Grape vines with cover crop (80% canopy) | 41 |
Note, the data in the table may be incorrect or outdated, and are intended as example for teaching purposes only. Reference values in this table have been compiled by faculty and staff at Cal Poly throughout the years, and the original references for the reference values have been lost. It is recommended to base irrigation scheduling on evapotranspiration rates and soil moisture monitoring.
- You are growing almond trees (Prunus dulcis), without a cover crop, in the Fresno area. The ECw of the irrigation water source is measured as 1.5 dS/m. Calculate the amount of irrigation water required on a seasonal basis for salinity control to maintain at least 90% maximum yield. You will need to calculate “Leaching Requirement” and “Applied Water”. Show your work in the text or upload a picture. Make sure to draw a circle around your final answer or make it bold.
This part of the lab assignment is to be completed during the in-person lab
Measuring pH in the field
In the lab, pH would be determine using a pH meter. For pH measurements in the field, we can use test strips. Test strips are less accurate than the pH meter, but they are relatively inexpensive and easy to use. Follow the instructions to determine the pH of your soil. We will measure soil pH in solution using a 1:2 ratio of soil to DI water.
- Identify the 2 soil samples your group collected in week 2
- For each soil sample, take a clean 50 ml plastic centrifuge tube and fill it with soil to the 10mL mark.
- Top up the falcon tube with 20mL deionized (DI) water. When you are done topping up, the water surface should be level with the 30mL mark on the falcon tube.
- Place the cap on the falcon tubes and shake for 30 seconds. Let the soil settle for 1 minute.
- Once the soil is settled, record the pH for each soil sample using a pH test strip.
pH buffering
Remember, we learned earlier that pH is the negative log of the hydronium concentration. Based on the problems you have solved in preparation of this lab, you should be able to calculate the theoretical pH of the your 1:2 soil solution, after you add 5mL of 0.1 M HCl (hydrochloric acid) using the following 2 equations and some algebra.
pH = -log[H+]
[H+]mix = ([H+]initial x Vinitial + [H+]HCl x VHCl)/(Vinitial + VHCl)
where 'mix' refers to the 1:2 soil solution, 'HCl' to the 1M hydrochloric acid, and 'initial' to the 1:2 soil solution before you added the HCl.
Let's see what happens in reality:
- Take your 1:2 soil solution for the tree row. Add 5 mL 0.1M HCl to the 1:2 solution of soil to deionized water. Use the graduation on the falcon tube to guage the amount of HCl added
- Place the cap on the falcon tube, shake for 30 seconds
- Let the solution sit for 5 minutes. Record the new pH with a pH test strip
Determination of Soil Salinity using the FieldScout Direct Meter
This instrument is designed for direct measurement of salts in soil media and solutions using a stainless steel probe. The measurement region is at the tip of the probe. Do not touch the sensor tip with your fingers. The oils on your skin will affect the probe’s measurement accuracy. Because the EC readings are affected by water content of the sample, it is important that soil moisture content does not vary significantly between readings. Your EC reading needs to be multiplied by a conversion factor to obtain the equivalent Saturated Media Extract (SME) value.
Procedure
- For each of your 2 soils, fill a clean falcon tube with soil to the 10mL mark.
- Using a wash bottle, gradually add deionized water and mix uniformly (free of partially wetted clumps) until a saturated paste is obtained.
- Fine textured soils (> 40% clay) may puddle easily. To minimize puddling and obtain a more definite endpoint with fine-textured soils, water should be added with a minimum amount of stirring, especially in the early stages of wetting. Some fine textured soils swell considerably upon addition of water. In these cases, step 2 must be repeated until the paste characteristics are stable.
- At saturation, the soil paste:
- Does not have free standing water on the surface of the paste.
- Soil paste slides freely and cleanly off a spatula (unless clay content > 40% clay).
- Paste will flow slightly when the container is tipped to a 45 degree angle from horizontal.
- Soil surface glistens as it reflects light.
- Consolidates easily by tapping after a trench is formed in the paste with the flat side of a spatula (may not apply to sandy soils >70% sand).
- When your sample is ready, press the On/Off button to power up the meter.
- Insert the probe tip into the saturated paste and read the results.
- Results are reported as milliSiemens per cm (mS/cm). 1 mS/cm = 1 deciSiemen per meter (dS/m). Record ECsp here ______mS/cm or dS/m. (ECsp is EC of the saturated paste)
- Turn the meter off.
- Rinse the probe free of soil with DI water.
- Calculate the EC of your soil (ECspe, equivalent to the EC of the saturated paste extract) using the following equation:
ECspe = 2.7 x ECsp + 0.8
- Note down the pH in the tree row and the tractor row. If you are joining virtually, one of the instructors will follow and film one of the in person groups while they are taking the pH measurement.
- Is the pH different in the tree row compared to the tractor row? Explain your observations.
- Calculate the anticipated pH, after adding 5mL HCl to your 2:1 water soil solution. Show your work. You only need to do the calculation for the soil from the tree row.
- Write down the soil pH after you added 5mL HCl to your 1:2 soil solution. Is the pH the same as the pH you calculated? Explain your observation.
- What is the EC in the tree row and the tractor row? Make sure to apply the equation to convert from EC in the saturated paste to EC equivalent in the saturated paste extract. Evaluate whether there is a salinity problem in the orchard. If you are joining virtually discuss with your peers on zoom what could cause differences in EC between the tree row and the tractor row.