Lab 4 Basic cations and sodicity
Lab 4 Basic cations and sodicity
The cation exchange capacity (CEC) comprises the total amount of cations that can be adsorbed onto the soil colloids. First, refresh your memory on why colloids hold on the cations.
Now, cations held on the cation exchange complex are in equilibrium with the soil solution and easily exchanged with the soil solution. In other words, they move easily in and out of the soil solution and are readily available to plants. Meanwhile, they are held strongly enough to prevent them from leaching below the root zone. Given that many essential plant nutrients occur in soils as cations, one could say that the cation exchange complex serves as a reservoir for plant nutrients. In western soils, the dominant cations on the cation exchange complex are Ca2+, Mg2+, K+ and Na+. These cations are called non-acid or basic cations. In regions with higher rainfall, H+ and Al3+ can constitute a significant portion of the CEC, especially in older soils. Remember, H+ and Al3+ are considered acid cations. Al3+ is an acid cation because it splits water and precipitates with OH-, leaving H+ behind in the soil solution.
Because cation exchange capacity is associated with the amount of negative charges found on colloids, the total cation exchange capacity of a soil will depend on the amount of colloids, as well as the type of colloids in that soil.
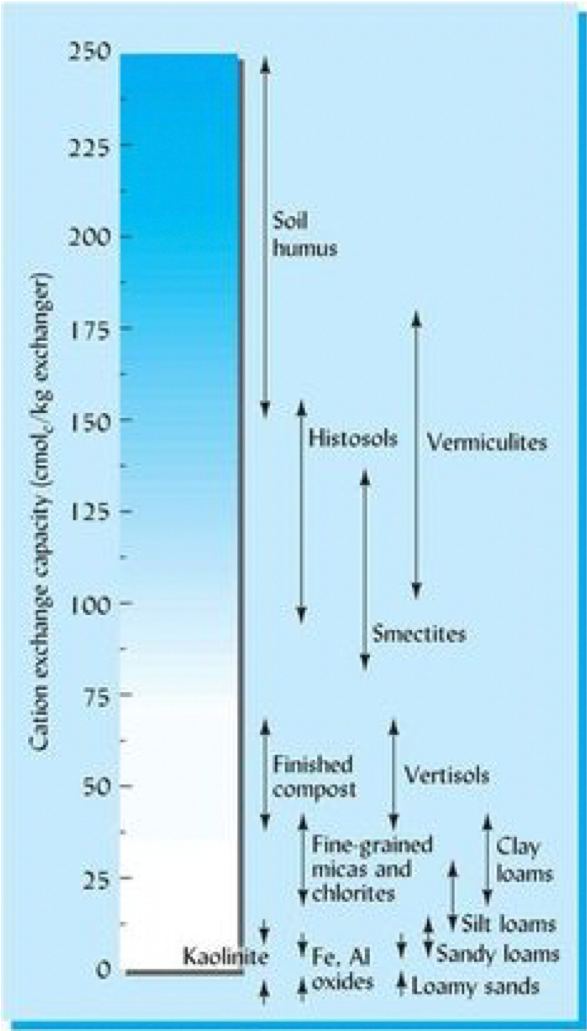
Soil organic matter has a very high CEC per unit colloid, while kaolinite and iron and aluminum oxides have a relatively low CEC per unit colloid.
Let's do some hypothetical math problems to see how the colloid composition of the soil affects CEC. Assume the following CEC values for different colloids:
- Humus or soil organic matter (SOM): 200 meq/100g SOM
- Vermiculite: 115 meq/100 g vermiculite
- Montmorillonite: 90 meq/100 g montmorillonite
- Illite: 35 meq/100 g illite
- Kaolinite: 8 meq/100 g kaolinite
Example calculation
Assume you have a soil with 20% illite clay and 5% soil organic matter, what is the CEC of your soil?
CEC = 0.2 g illite/g soil x 35 meq/100 g illite + 0.05 g SOM/g soil x 200 meq/100 g SOM = 17 meq/100 g soil
You may have noticed that the unit for cation exchange capacity is meq/100 g soil. Go to the next section to learn what this unit means!
- In your own words, describe why soil colloids can hold onto cations
- What are the dominant cations on the cation exchange complex in western soils?
- A soil contains 3.5% organic matter and 10% vermiculate clay. If these are the only sources of cation exchange in the soil, what is a reasonable estimate of its CEC in meq/100g of soil? Show your work!
- If you raise the SOM content in a soil with 10 percent illite from 2 to 4% SOM, how much would the CEC of that soil increase, assuming that SOM and illite are the only colloids in the soil? Show your work!
- Based on your answer for the previous question, what can you say about the role of SOM in CEC? Is the increase in CEC significant?
Typically, soil testing labs will determine the cation exchange capacity by summing all cations extracted from the soil. In this stimulus, you will learn how cations are extracted from the soil, and how these lab results can be used to calculate cation exchange capacity.
In the previous virtual lab assignment, you already learned how to determine the amount of acid cations in the soil, namely, by extracting cations with KCl, followed by a titration with NaOH and phenolphthalein as indicator. Now, we need to also extract the basic cations, to determine the total cation exchange capacity. We know that K+ is one of the dominant cations on the cation exchange complex. Therefore, KCl would not be a good extraction solution for basic cations. We need to find an extraction solution that does not contain any of the elements we are interested in measuring. Scientists decided that ammonium acetate (NH4OAc) is a good candidate for this task.
How do soil extractions work, conceptually? Watch the video!
Once you know the concentration in your extract, you have to do a few calculations to get the concentration in your soil. You do this multiplying your nutrient concentration in your extract (in ppm or mg/L) by the volume of your extraction solution (in L), and dividing by the mass of soil you weighed out initially (in kg). This gives you ppm of your nutrient in your soil, or mg of nutrient per kg of soil.
Now, how do you get from cation concentrations in ppm or mg/kg soils to cation exchange capacity, in meq/100 g soil? How do you calculate the cation exchange capacity of your soil, and how do you determine base saturation? Learn about it in this video.
Shortcut equations to convert between meq/100 g soil and ppm
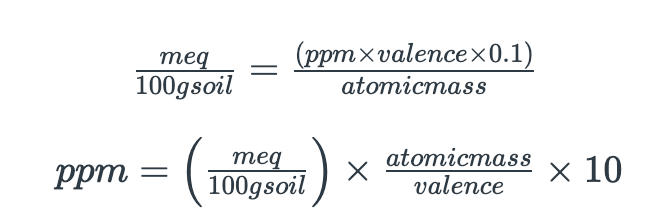
Remember, 100 is part of the unit 'meq/100 g soil'. Do NOT divide by 100!
- Describe in your own words the concept of soil extraction
- Which extraction solution is typically used to determine basic cation concentrations?
- In your own words, describe the unit for cation exchange capacity, namely meq/100 g soil
- If a soil has 30 meq Ca/100 g soil, 5 meq H/100g soil and 10 meq Mg/100g soil, how much ppm Ca, H and Mg does this come down to? Show your work!
- If a soil's CEC is 20 meq/100 g soil and a Mg saturation of 20%, what is the ppm Mg in this soil? Show your work!
- You are provided with the data in the table below for your soil. Given this information, determine the CEC, base saturation, and exchangeable sodium percentage.
Cation meq/100 g soil Ca2+ 20 Mg2+ 6 K+ 0.6 Na+ 0.1 Al3+ 0 H+ 0.19 CEC
Nutrient recommendations vary from crop to crop and region to region. The following recommendations for basic cations are excerpted from the University of California's Vegetable Research and Information Center 2007 publication by Tim Hartz entitled "Soil Testing for Nutrient Availability Procedures and Interpretation for California Vegetable Crop Production".
Potassium recommendations for California vegetables
Vegetable crops vary widely in K uptake, and therefore have different soil K requirements. In addition to the crop to be grown, other factors affecting soil test K interpretation are soil physical characteristics, the relative abundance of other soil cations, and irrigation practices. Rooting density greatly affects a plant’s ability to extract K from the soil; any soil characteristic (subsurface compaction, poor structure, etc.) that limits rooting density or depth will restrict K uptake. Other soil cations can compete for plant cation uptake; therefore, soil K availability should be evaluated both on a ppm basis, and as a percentage of exchangeable soil cations (on a meq basis). Cation competition is generally not a problem in soils in which K makes up > 3% of exchangeable cations, whereas soils in which K makes up < 2% of exchangeable cations may have restricted K availability, even with relatively high exchangeable K levels. Lastly, any irrigation practice that concentrates rooting in a small area (i.e. drip irrigation) may restrict soil K uptake compared to an irrigation approach that wets the entire soil volume. The following chart contains generalized recommendations based on ppm exchangeable soil K.
Ammonium acetate extractable K threshold levels (in ppm) for crop response to K fertilization. Threshold values are adopted from Hartz 2007.
| Crop | Crop response likely | Crop response possible | Crop response unlikely |
| Celery | < 150 | 150-200 | >200 |
| Other cool-season vegetables | <100 | 100-150 | >150 |
| Potato, tomato, pepper | <150 | 150-200 | >200 |
| Cucurbits | <80 | 80-120 | >120 |
Calcium and magnesium recommendations for California vegetables
Nearly all California soils contain sufficient Ca and Mg to meet the nutritional requirements of vegetable crops. Calcium typically accounts for 40-85% of exchangeable cations on a meq basis, while Mg accounts for 10-50%; in most soils the amount of Ca and Mg on soil exchange sites is much greater than that necessary to satisfy crop uptake requirements. Physiological disorders such as blossom end rot of tomato and blackheart of celery still occur, but generally not from lack of available soil Ca. In most cases Ca-related physiological disorders are caused by water stress (which disrupts Ca uptake and transport within the plant), or heavy NH4+ fertilization (which suppresses Ca uptake); soil Ca application is seldom effective in reducing these disorders.
Ca : Mg ratio is often included on soil test results. This parameter is useful primarily as a guide to soil structure rather than nutrient availability; higher Ca : Mg ratios are associated with better soil structure, tilth and water infiltration rate. As previously stated, available soil Ca and Mg is best estimated by a saturated paste extraction, not an ammonium acetate extraction. Soils with a saturated paste Ca : Mg ratio less than 2:1 (meq basis) may benefit from Ca application (typically as gypsum or lime), although the cost may outweigh the agronomic benefit.
- Your soil has a calcium saturation of 60% and a magnesium saturation of 20%. The soil test reports shows a potassium concentration of 75ppm. You are planning to grow tomatoes. Following the guidelines excerpted from the University of California Vegetable Research and Innovation Center, would you include any basic cation nutrition in your fertilizer program? Explain.
After our discussion of chemical soil properties in lecture, you may still be a bit fuzzy on concept of sodicity. Here, we'll explore further what sodicity is, how sodicity affects the soil and the plant, and how to fix a sodicity issue. First, let's have a look at how sodicity affects soil structure and water movement in the soil.
To diagnose a sodicity problem, you want to run a soil test and determine the exchangeable sodium percentage or sodium adsorption ratio. However, there may be some tell tales in your field to suspect a sodicity issue. In the next video, you will see some sodium affected soils, and some proposed treatments to rehabilitate the soils.
Managing sodicity
Several commercial products are now on the market for amending sodic and saline-sodic soils. The only function of scientifically proven amendments is to provide soluble calcium to replace exchangeable sodium adsorbed on clay surfaces. There are two main types of amendments: those that add calcium directly to the soil and those that dissolve calcium from calcium carbonate (CaCO3) already present in the soil.
Calcium amendments include gypsum (CaSO4•2H2O; hydrated calcium sulfate) and calcium chloride. Gypsum is moderately soluble in water. Calcium chloride is highly water soluble and fast acting, but is generally too expensive for most situations.
Gypsum is a highly available, easily applied source of Ca ions. Recovering one acre-foot of sodic soil requires approximately 1.7 tons of pure gypsum (CaSO4•2H2O) for each milliequivalent of exchangeable Na present per 100 grams of soil.
There are acid-forming, or acidic amendments that dissolve calcium from calcium carbonate (CaCO3) already present in the soil. Sulfuric acid reacts immediately with the soil CaCO3 to release soluble calcium for exchange with sodium. Elemental sulfur must be oxidized by soil bacteria and react with water to form sulfuric acid. The formation of sizeable amounts of sulfuric acid from elemental sulfur may take several months to several years. Choice of amendment is mainly based on the cost of the soluble calcium furnished directly or indirectly by the amendment and by the speed of the reaction.
Example gypsum requirement calculation
Your soil has a CEC of 18 milliequivalents per 100 grams and Exchangeable Sodium Percentage of 26%. You wish to apply enough gypsum to reduce the ESP to approximately 10%. How much gypsum do you need to apply per acre-foot of soil to do the job?
ESP of 26% – desired ESP of 10% = ESP of 16, or 16% exchangeable Na must be replaced with calcium (Ca) to achieve the desired ESP.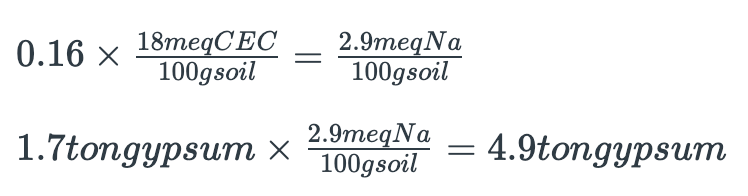
Thus, about 5 tons of pure gypsum per acre would be required to reclaim the top 12 inches of this soil. This calculation should be adjusted for lower grades of gypsum and different soil depths.
- Explain, in your own words, how and why sodicity affects water infiltration
- What are some visual observations you notice in the sodium affected soils shown in the video
- List 3 treatments the researchers are exploring to remediate the sodium affected soils
- Your soil has a CEC of 25 milliequivalents per 100 grams and Exchangeable Sodium Percentage of 18%. You wish to apply enough gypsum to reduce the ESP to approximately 10%. How much gypsum do you need to apply per acre-foot of soil to do the job. Assumption: Recovering one acre-foot of sodic soil requires approximately 1.7 tons of pure gypsum (CaSO4.2H2O) for each milliequivalent of exchangeable Na present per 100 grams of soil. Show your work!
This part of the lab assignment is to be completed during the in-person lab
You have learned that soil K concentrations are typically determined in the laboratory based on an ammonium acetate extraction, followed by analysis of the extract using and AES-ICP. Alternatively, some labs will determine basic cation concentrations on saturated paste extracts. How well do the different methods to determine cation concentrations in soil compare? Let's have a discussion as a class group. Don't forget to write down your answer!
In Spring 2020 shortly after mowing the cover crop, we collected soil samples in the lemon orchard trial. We'll have a look at the data for basic cations and discuss patterns and trends. Don't forget to answer the relevant questions on the right.
- How do cation concentrations measured by ammonium acetate extraction and saturated paste extractions compare? Why?
- How o location and cover crops impact Ca, Mg, K and Na concentrations at the Cal Poly lemon orchard?
- Consult the CDFA fertilizer guidelines to assess whether your soil potassium concentration is sufficent for citrus cultivation
This part of the lab assignment is to be completed during the in-person lab activity
We saw in the videos how addition of sodium dispersed the clay particles, and addition of gypsum flocculated clay particles. Let's see if we can disperse and flocculate the soil from the lemon orchard.
- Add a photo of the soil in the different treatments from the time differences appear the greatest
- Describe your observations for each of the 10 treatments
- Why would there be differences between DI water and tap water?
- How do low sodium chloride and gypsum additions affect flocculation and dispersion, why?
- How do high sodium chloride and gypsum additions affect flocculation and dispersion? Why?
- In your own words, describe how gypsum mediates a sodicity problem.
This part of the lab assignment is to be completed during the in-person lab activity
The quality of irrigation water can have a big effect on water infiltration. Infiltration is one of the reasons to check irrigation water quality before applying it to a field. Besides the quality of the irrigation water, infiltration is also affected by soil health, especially soil structure. Here, we will test the effect of irrigation water quality and soil health on infiltration using infiltrometers and four different water treatments, namely:
- Tap water
- DI water
- DI water + low addition of NaCl
- DI water + low addition of CaSO4
We will do the test in the area of the orchard where no cover crop was planted, compared to the area where a cover corp was grown.
We will measure infiltration rate in the field using a double ring infiltrometer method. In short, two concentric rings are hammered into the soil surface. The outer ring is filled with tap water to control lateral flow. Infiltration rate of each solution will be determined by pouring a known volume of the solution in the inner ring and timing how long it takes for the solution to infiltrate. Then, infiltration rate is calculated as
Infiltration rate = (volume)/(time elapsed)
We will choose a volume of 100mL to be poured into the inner ring. The time elapsed is the time you record.
Infiltration rate decreases with increasing soil moisture content. As such, the infiltration rate will decrease as time intervals progress, until a steady state is reached. Therefore, we will determine infiltration rates for 5 consecutive times.
Fill out the worksheet provided.
- Discuss how infiltration rate is affected by water quality and soil health. Include in your discussion potential explanations for your observed results
- Based on what you learned from this experiment, what would you do if you observed poor infiltration in your almond orchard nearby Fresno?
