Lab 6 Phosphorus, Sulfur & Introduction to Nutrient Management
Lab 6 Phosphorus, Sulfur & Introduction to Nutrient Management
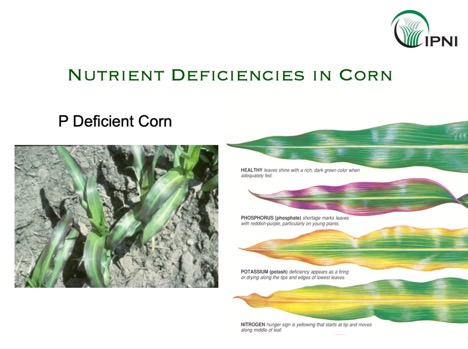
Phosphorus (P) plays a role in many plant functions. Most notably, P is an important constituent of DNA and a building block of Adenosine triphosphate (ATP), essential for energy storage and transfer. Symptoms of phosphorus deficiency in corn are generally observed by purple discoloration on the leaves.
Forms of P and challenges related to P availability
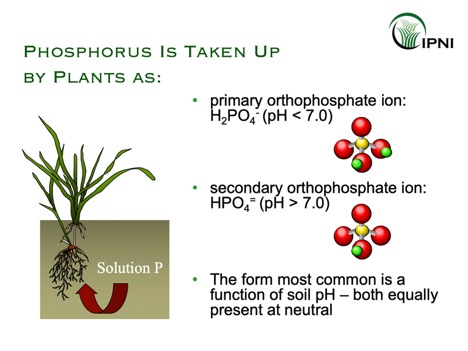
The plant available form of phosphorus in the soil is orthophosphate. Orthophosphate concentrations in the soil solution are generally low, and account for less than 1% of the total amount of soil P.
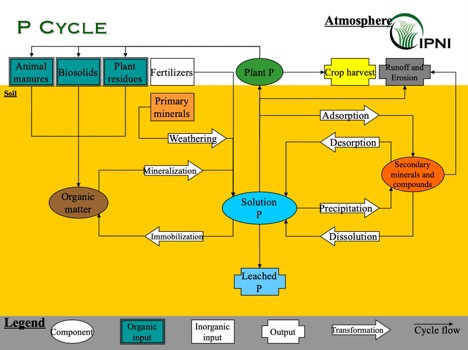
Where is P when it is not in soil solution? To answer this question, we have to take a closer look at the P cycle. When P fertilizer is applied to the soil, orthophosphate is readily adsorbed onto colloids, precipitated as secondary minerals, or immobilized by microorganisms. P can also be lost from the soil, mostly through runoff, but P leaching can be important under some conditions.
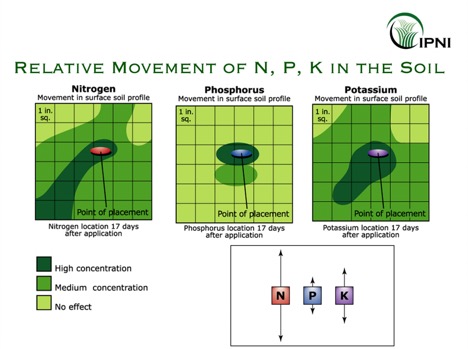
Processes exist to release P from these plant unavailable pools, including dissolution, desorption and mineralization. However, the balance between precipitation vs. dissolution, adsorption vs. desorption, or immobilization vs. mineralization tends to be shifted towards making P plant unavailable. Because of that, P availability and mobility in the soil is typically low.
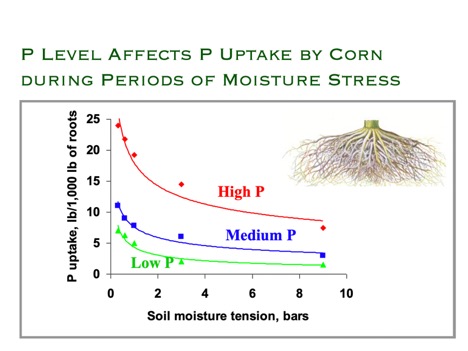
The low availability of P is further exacerbated by soil moisture content. The ability of the plant to take up phosphorus strongly decreases at low soil moisture content.
Because of the challenges associated with keeping soil P in a plant available form, P fertilizer use efficiencies are typically low. As a global average, only about 30% of phosphorus applied as fertilizer is taken up by the crop.
Environmental impacts related to P loss
We hear about climate change in the media relatively often, we hear about biodiversity loss, more and more are we made aware of the urgency for tackling nitrogen pollution, but an issue that receives far less attention is phosphorus. Learn in the video below the challenges we are already facing and are expected to become worse in years to come.
In addition to the solutions proposed in the previous video, research have proposed to improve the phosphorus use efficiency in agricultural system and reduce phosphorus loss to the environment by taking advantage of our understanding of soil ecology and biogeochemistry, most notably the highly specialized skills of our soil biota. In this regard, a promising avenue is to promote symbiosis between plants and mycorrhizal fungi. The next video walks you through how mycorrhizal fungi can help solve the P crisis.
P extraction methods
Various methods have been developed to assess the amount of plant available P in agricultural soils. In California, we will typically use Olsen P. Learn why in the video below.
Understand how to interpret P test results
Soil P tests extract an operationally defined fraction of P from the soil. These extracts include the P present in soil solution at the time of soil sampling, plus a certain amount of P desorbed from colloids or dissolved from secondary minerals. Extraction solutions are carefully selected to match soil chemistries and mimic the P pool that may become available to the plant during the growing season. Despite our best efforts at selecting extraction chemistries that match regional soil characteristics, these soil P tests are merely our best guess at how much P may become plant available, and can not be interpreted as a quantitative assessment of plant available P in the soil. In reality, different crops have different efficiencies for P uptake, and may therefore experience the soil environment with respect to P availability in different ways.
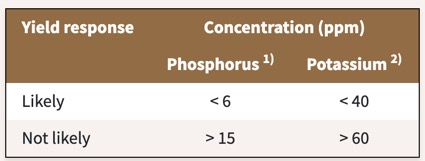
Well, that's confusion! What does that mean for the interpretation of soil P test results? Agronomists have conducted research trials to assess the likelihood that crops will respond to additional P fertilizer in function of the soil P test levels present in the soil. Let's take the example of Barley.
Research findings indicate that if Olsen P levels in the soil are less than 6 ppm, barley is likely to show a positive yield response to fertilizer P. If the Olsen P levels are greater than 15, barley yield will unlike increase with P fertilization. This implies that if Olsen P test levels are less than 6ppm, you should apply P fertilizer to your barley. The amount of P you apply in that case equals the amount of P expected to be taken up by the crop, corrected for the anticipated phosphorus use efficiency. For example, if you expect your crop to take up 15 lbs P/acre, and you expect the P use efficiency to be 30%, you would apply 15/0.3 = 50 lbs P/acre. If Olsen P levels are over 15ppm, you should not apply P fertilizer to your barley. What if Olsen P is between 6 and 15ppm? In that case, you are recommended to apply the amount of P taken out of the field by the harvested crop. The California Department of Food and Agriculture (CDFA) provides threshold levels for interpreting P test results are provided for many crops commonly grown in California in the CDFA fertilizer guidelines. To find info for your crop, select the crop, scroll down to phosphorus, select soil test, and subsequently select interpretation of soil test report.
- What is a typical sign of phosphorus deficiency?
- What is the plant available form of P?
- Why is phosphorus availability in soil often very low?
- How does soil moisture content affect plant P uptake?
- Where does P fertilizer come from?
- Which environmental problems does P cause?
- Provide some solutions to address issues related to both the access to P fertilizer and the environmental issues related to P loss.
- Mycorrhizae form a symbiotic relationship with the host plant. In your own words, describe the difference between a symbiont and a pathogen
- Which 3 P tests are mentioned in the video produced by New Mexico State University?
- Which P test is recommend for Western soils, and why?
- Which extraction solution is used in Olsen P?
- You are planning to grow carrots. Your soil test report indicates that your soil contains 20 ppm P. Will you apply P fertilizer? Justify your answer.
Know the forms of S available to plants
Just like other nutrients, sulfur can take various forms in the soil. Refresh your memory of the S cycle by watching the video below.
Standard methods for S determination in soil
The standard method for the determination of plant available S in soil is based on an extraction with Ca(H2PO4)2.H2O with subsequent determination of SO42--S by turbidimetric measurement. Calcium phosphate is utilized to suppress the dissolution of organic matter and for the removal of sulfate that maybe absorbed. Turbidimetric analysis is based on the formation of BaSO4 crystals in a suspension and subsequent measurement of optical density (Soil, Plant and Water Reference Methods for the Western Region, Miller et al. 2013). The turbidimetric method requires practice to become proficient and is sensitive to high concentrations of dissolved soil organic carbon. Because of the variability and uncertainty associated with this method, some laboratories have switched over to using inductively coupled plasma atomic emission spectrometry (ICP-AES) rather than the turbidity method. Note that ICP-AES measures total S in an extract, whereas the turbidity method only targets sulfate-sulfur. Read the following article to learn about how the 2 methods compare. Some labs use lithium chloride rather than Ca(H2PO4)2.H2O to extract sulfate.
How to interpret S values on a soil test report
A&L labs rates SO42--S levels in soil as follows:
- < 3 ppm S: Very low
- 3-10 ppm S: Low
- 10-25 ppm S: Medium
- 25-35 ppm S: High
- 35-60 ppm S: Very high
Dellavale Labs provides crop-specific threshold values, similar to the interpretation of P test results, for a select number of crops.
S threshold levels, in ppm
| Crop | Response likely below | Response unlikely above |
| Alfalfa | 5 | 10 |
| Wheat and Barley | 5 | 10 |
| Pasture and range | 5 | 10 |
Note, commercial soil testing labs update reference values as new data becomes available, and reference values included here may not be the most current reference values used by the labs.
In general, interpretation guidelines for California crops are much less established for S compared to other macronutrients such as N and P.
Sulfur deficiency in a California case studies
Stone fruit
The Division of Agriculture and Natural Resources of the University of California (UCANR) published nutrient management guidelines for stone fruit orchards. They note that S deficiencies have been been documented in California stone fruit orchards to date. Find out more on UCANR Fruit Report.
Alfalfa
Sulfur deficiencies in alfalfa have been observed in the Sacramento Valley and the east side of the San Joaquin Valley. Sulfur deficiencies can only be confirmed by plant tissue testing. To address sulfur deficiencies, one can apply a slow acting, long term, S source or a fast acting, short term S source. Learn more about managing S deficiencies in the UCANR publication on irrigated alfalfa management.
- What is the plant available form of sulfur?
- List 2 differences and 2 similarities between the N and the S cycle
- Explain, in your own words, the main difference between the turbidity based method versus the inductively coupled plasma method. How do the 2 methods compare?
- You are planning to grow wheat and your soil test report comes back with a sulfate-fulfur concentration of 15ppm. What is your interpretation? What will you do with regard to S fertilization?
- Why are S deficiencies uncommon in California stone fruit orchards?
- Provide specs or examples for short and long-term S sources for alfalfa.
This activity is to be completed during the in-person lab activity
Fertigation - the concept is simple: applying fertilizer through the irrigation system. But how does it work in practice?
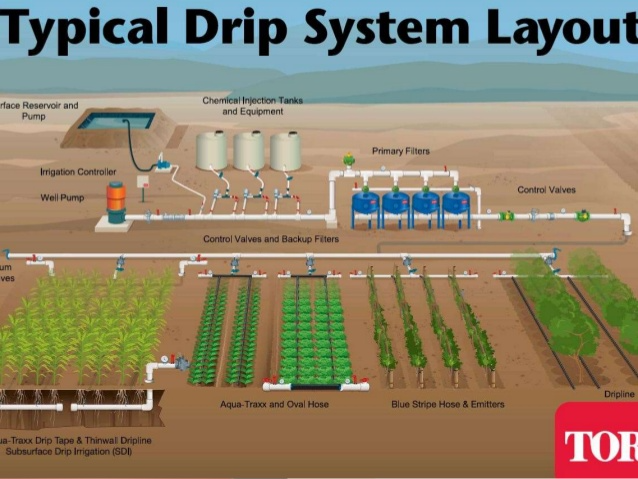
We will visit with Johnny Rosecrans, Cal Poly's orchard manager, to learn more about how he applies fertilizers to the various tree crops grown at Cal Poly.
- Describe two ways to apply fertilizer through the irrigation system: (1) an old-school way of manually injecting the fertilizer and (2) a high tech automated system for fertilizer delivery.
- Which fertilizers are in the fertilizer tanks at the crops unit? List the full name and the plant essential nutrients contained in each fertilizer source.
- Compare and contrast the low-tech with the high-tech system of fertilizer delivery by listing at least 3 advantages and/or disadvantages.
- Which are typical nutrient deficiencies observed at the orchards at Cal Poly?
- List advantages and disadvantages of foliar applications versus delivering fertilizer through the irrigation system.
Each group will perform 4 extractions, 2 for the tree row and 2 for the tractor row. For each location, you will do one extraction with DI water, and one extraction where you first acidify the soil.
Procedure:
- Set up 4 centrifuge tubes labeled as follows
- Tree row DI extraction
- Tree row HCl extraction
- Tractor row DI extraction
- Tractor row HCl extraction
- Weigh 10 g of soil from the tree row in the tubes labeled tree row and 10 g of soil from the tractor row in the tubes labeled tractor row
- Dispense 30mL of DI water in each tube labeled DI extraction
- Dispense 5mL of 0.1M HCl and 25mL of DI water in each tube labeled HCl extraction
- Cap and shake all tubes for 30 seconds
- Let tubes sit until soil settles in the tip of the tubes
- Set up 4 centrifuge tubes with funnel and filter paper labeled:
- Tree row DI extraction
- Tree row HCl extraction
- Tractor row DI extraction
- Tractor row HCl extraction
- Filter each extract into the corresponding clean centrifuge tube
- Measure and record P concentrations in each of the 4 extracts with a P test strip.
The measured P concentration in the concentration in the extract. We want to know the P concentration in the soil. The P concentration in the soil can be calculated as follows:
Step 1: Convert ppm PO42- to ppm P
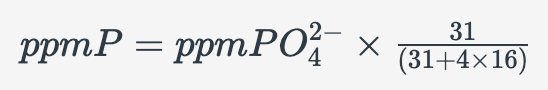
Step 2: Convert ppm P in extract (mg P/L extract) to ppm P in soil (mg P/kg soil)

Let's see what will happen... Use the figure below to guide the interpretation of your results.
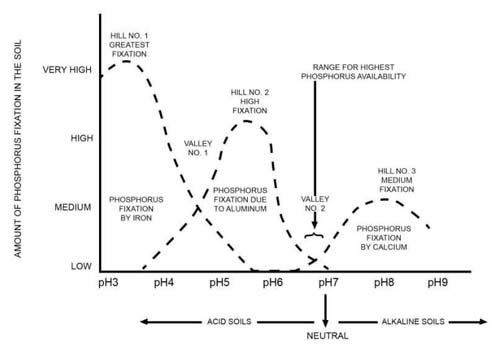
Note, this activity is meant to demonstrate the behavior of P in the soil and challenges associated with measuring soil P. This activity does not suggest that DI and HCl extractions are recommended for measuring P in the soil.
- Record the soil P concentration in the tree and the tractor row with versus without acid addition. Note, you will need to record 4 values
- Do you see any differences in the DI extraction between the tree row and the tractor row? What may explain your results
- Do you see any effect of the acid treatment? What may explain your results?
- How do you think the results from the DI and DI + HCl extraction would compare to Olsen P extraction? Explain your result.
This activity is to be completed during the in-person lab period
Remember we discussed the P index in lecture. The P index is a tool to determine the risk of P pollution from agricultural fields. Let's apply the P index to the case study of the lemon orchard.
Step (1), record you score for each of the transport and source factors.
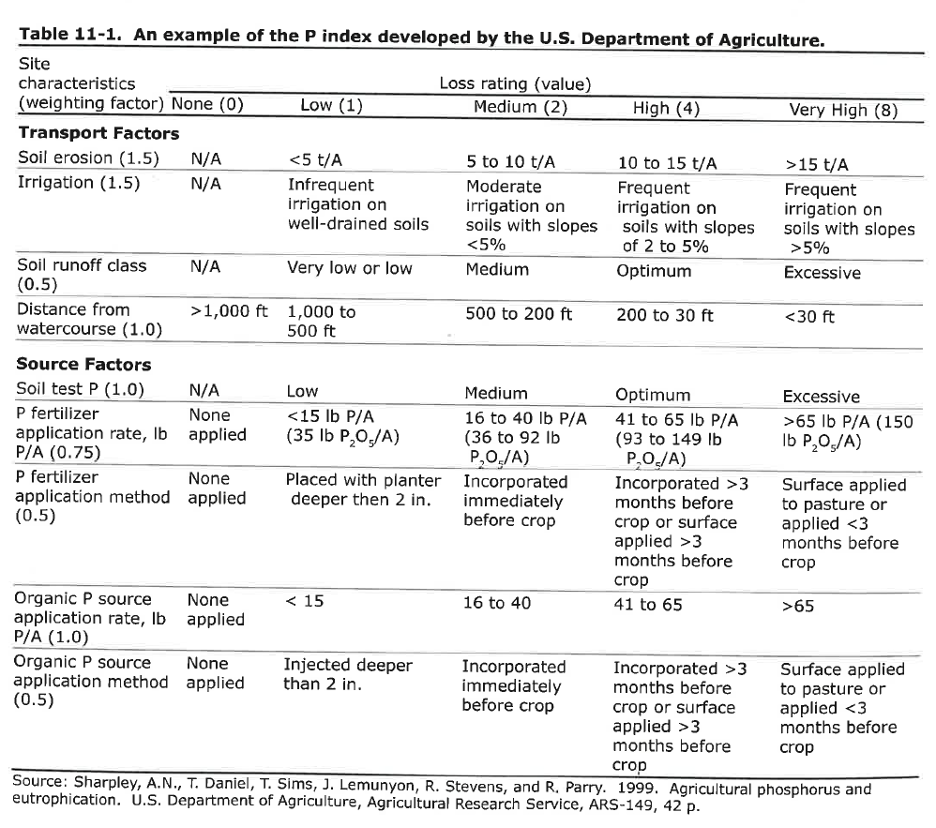
Step (2), multiply scores for each factor with the weight associated with that factor and sum the products. Look up your risk in the table below.
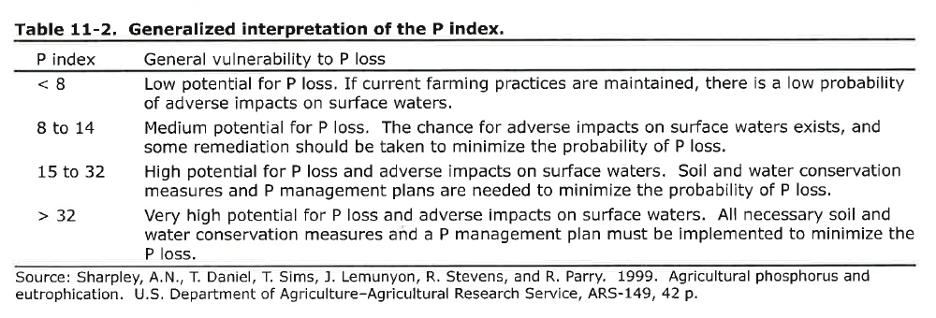
- Record the P index you determined for the lemon field. Show your math
- What is the generalized interpretation for the P index you calculated?
- In your own words, describe your action plan that matches the interpretation of you P index. what management changes would you propose, if any. Justify your answer.